Patients wanted for study on Post-COVID syndrome
Evaluation of antiviral therapy in patients with post-COVID syndrome
Randomised Adaptive Assesssment of post COVID syndrome treatments_Reducing Inflammatory Activity in patients with post COVID syndrome (RAPID_REVIVE)
The RAPID_REVIVE study is investigating whether drug treatment with an as yet unapproved drug has a positive influence on the development of Post-COVID syndrome.
The majority of patients recover from a COVID-19 infection without permanent impairment, but a significant proportion suffer from long-term consequences, known as Post-COVID syndrome. These people have symptoms for months. The symptoms can vary greatly and the causes are not yet fully understood. Symptoms range from shortness of breath, loss of smell and taste, fatigue, reduced resilience, muscle, head and joint pain to cognitive disorders, depression and anxiety. The severity of the symptoms can range from only limited impairments in the private and professional environment to complete incapacity to work with a significantly reduced quality of life. To date, there is no approved therapy and only the various symptoms are treated.
The RAPID_REVIVE study is investigating whether drug treatment with an as yet unapproved drug has a positive influence on the development of Post-COVID syndrome. To this end, the new drug is being compared with a placebo as part of the study.
Age: at least 18 years old
Participation is open to patients with a confirmed medical diagnosis of post-COVID syndrome that has already lasted more than 12 weeks.
Further requirements for participation:
- Moderate to severe physical limitation
- At least 2 of the following post-COVID symptoms must apply
- Fatigue (exhaustion)
- Cognitive impairment
- Shortness of breath
- Orthostatic/autonomic dysfunction (drop in blood pressure in upright positions)
- Ability to understand and follow the nature of the study and associated procedures
- Ability to provide and use a smartphone (or tablet) to install the software
- Correct and consistent use of a highly effective contraceptive method during the study
There are further requirements for participation, which will be explained in a personal discussion between medical staff and patients. Possible participation in the study must always be checked in advance by the medical staff.
Experience has shown that the maximum distance between the patient's place of residence and the trial centre should not exceed 50 km, as patients have to come to the trial centre several times for visits during participation in the trial.
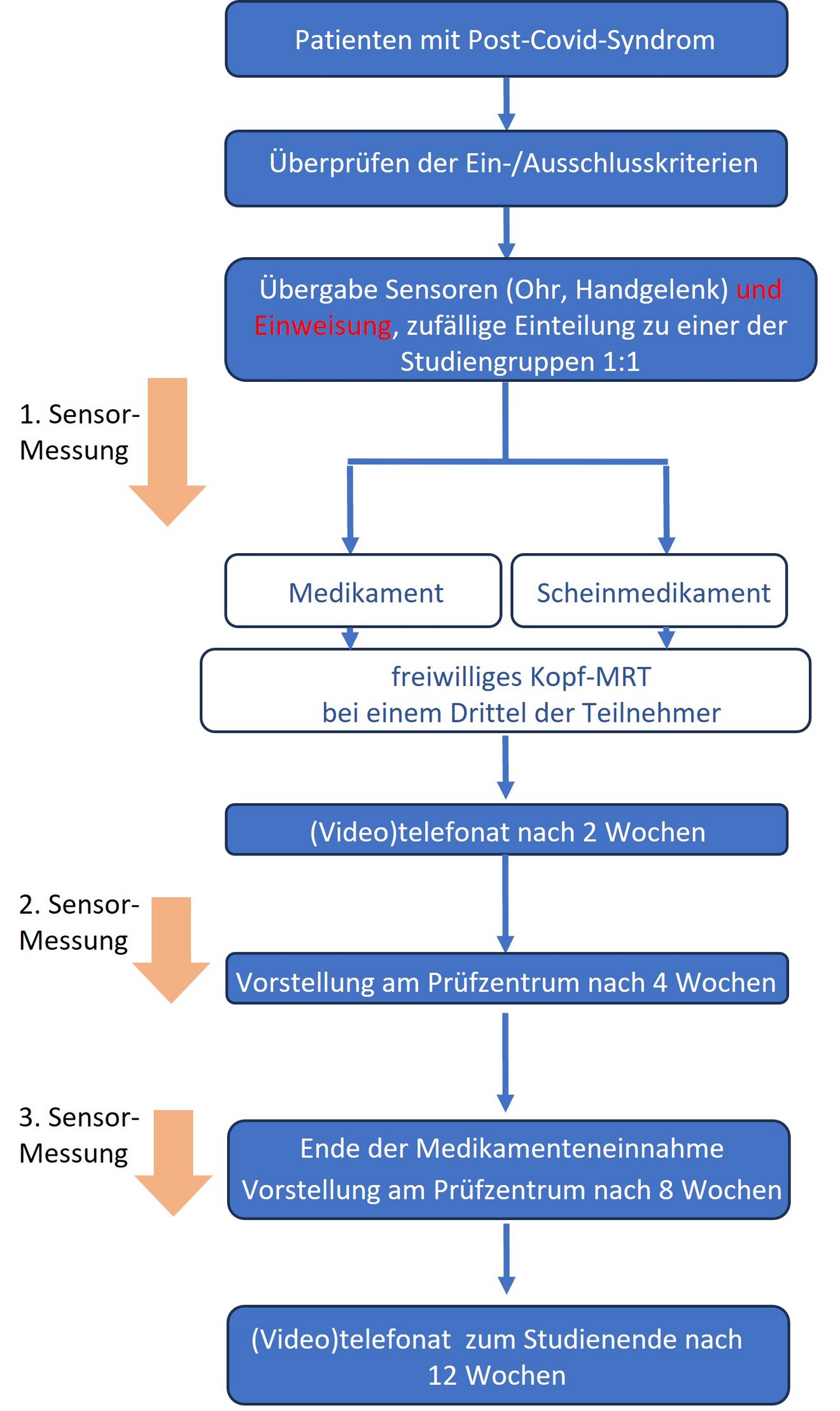
Procedure of the study
Patients are randomly assigned to one of the two study groups (drug / placebo). One group receives the drug treatment with the new medication, the other group receives a placebo without an active ingredient. Neither the medical nor the nursing study staff know which patients receive the drug and which receive the placebo.
Information on the physical and cognitive condition of both groups is collected via questionnaires. In addition, measurement data/vital parameters (e.g. oxygen saturation in the blood or pulse rate) are recorded using aids (smartwatch and ear sensor).
Participation in the study lasts about 3 months. There will be a total of 5 personal appointments at the test centre. During this time, participants take one tablet a day for 56 days. Two weeks after starting to take the tablet, a (video) telephone call is made to the medical staff at the trial centre.
This is followed by two personal visits to the trial centre after 4 and 8 weeks respectively. During the visits to the trial centre, medical check-ups, the collection of questionnaires, blood and stool samples and the dispensing of study medication will take place. A final (video) telephone call is made 4 weeks after the last tablet intake.
The RAPID_REVIVE study is open to patients who are at least 18 years old, have received a confirmed Post-COVID syndrome diagnosis and fulfil the other above-mentioned participation requirements. In this study, there are detailed guidelines as to which patients may participate in the study. Potential patients are therefore carefully examined by medical staff before being included in the study and receive a detailed information session.
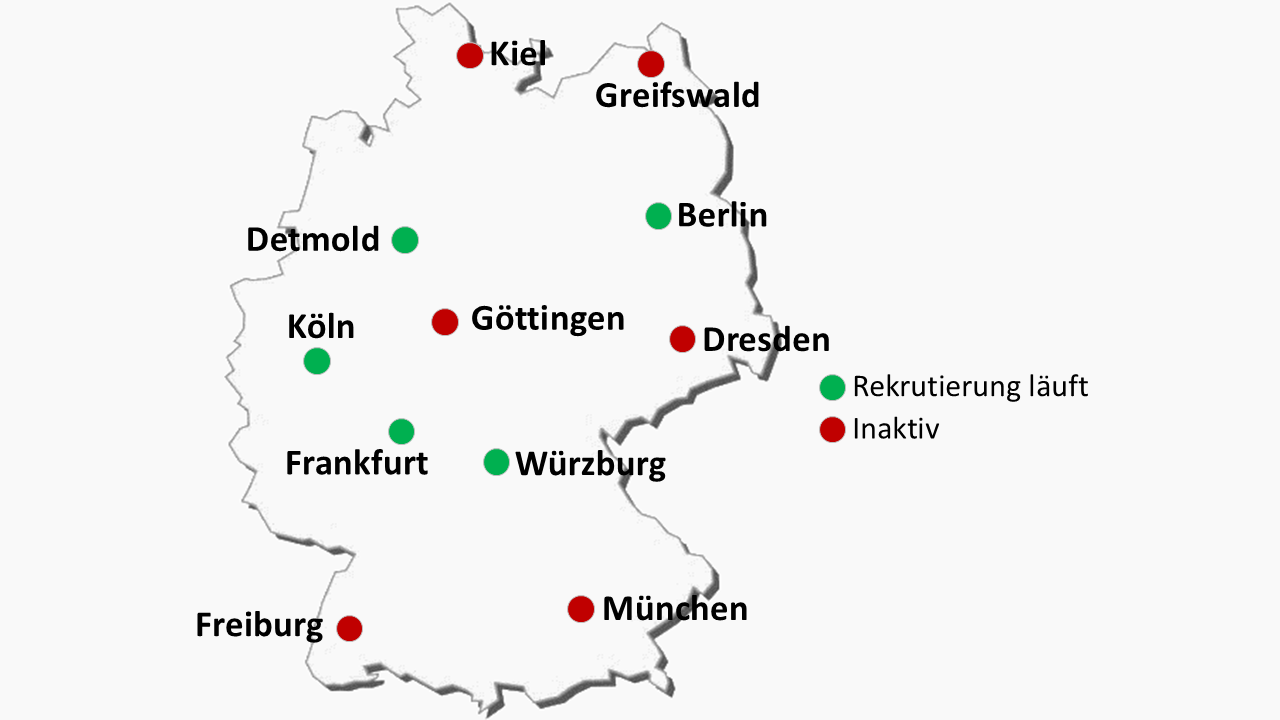
To participate, interested patients should live in the immediate vicinity (max. 50 km) of a trial centre. 11 locations in Germany shown on the map will participate in the future (see illustration of trial centres).
At present, not all sites have started to enrol patients. Interested patients can contact a trial centre near them (max. distance 50 km from their place of residence).
Only enquiries sent to the contact details below will be processed.
The use of all data and biomaterials collected in the study is subject to the strictest data protection guidelines. Each collection of data and biospecimens has been approved by an independent medical ethics committee. All data in the study that identifies individuals (name, date of birth, address, etc.) is encrypted. Individual patients are therefore not directly identifiable. All data is subject to the General Data Protection Regulation.
Subject insurance is also taken out for all participants in a clinical trial of a medicinal product.
This text is intended for medical personnel.